High Burden, Low Priority? Iron Deficiency Anemia in U.S Women and the Challenges of Data Gaps With Over-the-Counter Treatments

Low visibility of first-line treatment presents challenges in tracking iron deficiency anemia despite high prevalence and insufficient follow-up testing in this common condition.
Not all public health problems make the news: Iron deficiency may be among the more underreported and underdiagnosed public health concerns in the U.S. relative to its prevalence. As the world’s most common micronutrient deficiency, iron deficiency and the iron deficiency anemia it can lead to when left untreated have been considered a problem predominantly in developing nations. But both conditions are common in the U.S. Anemia can cause wide-ranging symptoms from restless leg syndrome, chest pain, weakness, and maybe even hearing loss, and can affect the body’s immune system. Recent research found that more than one third of U.S. females ages 12 to 21 have some form of iron deficiency, and the CDC estimates that one in six pregnant women in the U.S. has anemia or will develop it over the course of pregnancy, with far higher rates among Hispanic, Black, and Native American women. Untreated anemia in pregnancy can increase the risk of premature birth and is associated with both low birth weight and postpartum depression.
Testing and treatment for anemia are part of routine prenatal care, but outside of pregnancy, women may face more barriers to testing, diagnosis, and treatment. And there is also a barrier to the visibility of treatment for anemia in the data — the typical first-line treatment is supplementation with elemental iron in combination with dietary changes. While iron supplements are covered by most Medicare and commercial insurance plans, they are often paid for out of pocket and do not generate a claim, as they need no prescription, are low cost, and are widely available. These factors present challenges to understanding who is being treated for this condition and when.
 We wanted to explore the differences in treatment and follow-up for pregnant and non-pregnant women with anemia. We also wanted to highlight the importance of contextual data and proxies for treatments with low visibility. Using Komodo’s Healthcare Map™, we identified female patients newly diagnosed with iron deficiency anemia, limiting our cohort to those with no confounding factors that may increase the risk of iron deficiency, such as chronic kidney disease or a prescription for NSAIDs. Iron deficiency was identified with ICD-9/ICD-10 codes, and treatment was defined as prescriptions for ferrous sulfate, additional iron injections, or blood transfusions. Pregnancy was defined as any patient who had pregnancy-related codes within the two years prior to their diagnosis of iron deficiency anemia. Our analysis was run using a combination of Sentinel capabilities and Komodo’s Prism software atop our enterprise platform. Data on blood transfusions and results of lab work were sourced from Komodo’s new MapEnhance offering. Here’s what we found:
We wanted to explore the differences in treatment and follow-up for pregnant and non-pregnant women with anemia. We also wanted to highlight the importance of contextual data and proxies for treatments with low visibility. Using Komodo’s Healthcare Map™, we identified female patients newly diagnosed with iron deficiency anemia, limiting our cohort to those with no confounding factors that may increase the risk of iron deficiency, such as chronic kidney disease or a prescription for NSAIDs. Iron deficiency was identified with ICD-9/ICD-10 codes, and treatment was defined as prescriptions for ferrous sulfate, additional iron injections, or blood transfusions. Pregnancy was defined as any patient who had pregnancy-related codes within the two years prior to their diagnosis of iron deficiency anemia. Our analysis was run using a combination of Sentinel capabilities and Komodo’s Prism software atop our enterprise platform. Data on blood transfusions and results of lab work were sourced from Komodo’s new MapEnhance offering. Here’s what we found:
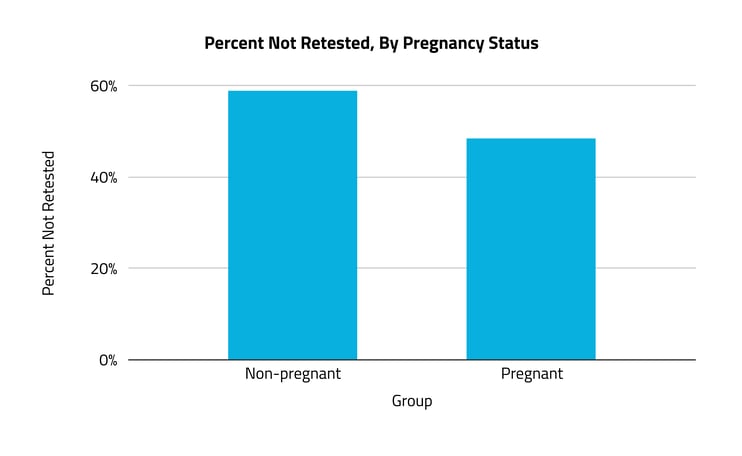
A majority of women with anemia did not receive follow-up testing in the six months after their diagnosis.
While anemia affects women of all ages, pregnancy is often a condition that necessitates treatment and close surveillance. Looking at claims for hemoglobin testing (one of the most widely used indicators for diagnosing anemia), we found that 59% of non-pregnant women with anemia did not receive a follow-up test in the six months after diagnosis. Among pregnant women, 48% did not receive follow-up testing in the same time period — an 11% difference.
Among non-pregnant women diagnosed with anemia, 77% did not receive a prescription for an oral iron supplement or intravenous treatment.
The remaining 23% of patients had a claim for either elemental iron supplements (ferrous sulfate) or an intravenous treatment. Numbers for treatment claims include both patients who did not receive treatment after a diagnosis and those advised to use over-the-counter elemental iron supplements. The high proportion of patients without a treatment claim may suggest that patients are paying for their own supplements, which, even at a low cost, increases barriers for some patients to sustain treatment.
We also found that pregnant women with iron deficiency anemia were 42% more likely than non-pregnant women to have a claim for either IV or oral supplements. Pregnant women were also more likely to receive IV supplementation.
Pregnant women are typically under closer clinical surveillance and more likely to receive prompt treatment for any conditions that may affect their pregnancy. However, considering the low rates of follow-up testing reported above, these findings may also reflect an undertreatment of anemia among both pregnant and non-pregnant women.
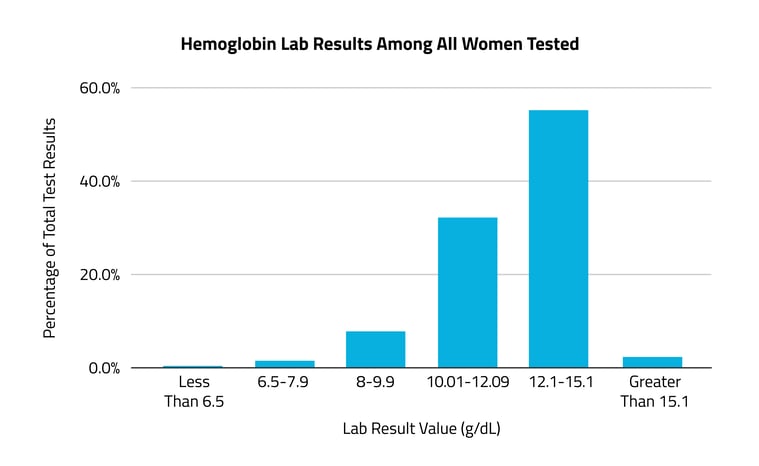
In the months to years prior to an anemia diagnosis, almost half (48%) of patients had at least one test result indicating low hemoglobin levels.
A hemoglobin test is one of the two most widely used to evaluate iron deficiency anemia. The normal range for females is 12.1-15.1g/dL. A concentration lower than this, and specifically lower than 11.9g/dL, is often used to diagnose anemia. Of all female patients diagnosed with anemia in 2021-2022 who had received one or more hemoglobin tests between 2018 and 2020, 48% had at least one result indicating low hemoglobin levels (less than 12.1g/dL). Among that population, 7% had at least one test result showing severely low levels (7.9g/dL or below), and 3% had at least one test result showing life-threatening levels (less than 6.5g/dL). The 52% with in-range hemoglobin levels likely received a test indicating iron deficiency closer to their diagnosis in 2021-2022.
These findings indicate the need for higher prioritization of anemia among non-pregnant women. While pregnancy is often a condition that necessitates treatment and close surveillance of anemia, this condition impacts women of all ages. Anemia has many downstream implications, so the same judicious surveillance should occur for pregnant and non-pregnant women alike.
These findings also highlight the low utilization of medical insurance for oral iron supplementation. While the majority of iron supplements are relatively low cost, any cost can increase the barrier to treatment adherence for some patients. Most preparations of iron are covered by insurance and can be prescribed and reimbursed, which would generate a claim. The low number of claims for over-the-counter supplements also creates low data visibility on treatment adherence, making it a challenge to capture a high-fidelity real-world understanding of anemia treatment and care.
When data gaps prevent stakeholders from gaining an accurate understanding of the on-the-ground reality, appropriate proxies and relevant contexts become even more key. Here, we were able to use claims for blood tests and time-stamped lab results sourced from Komodo's MapEnhance data product to sharpen our view of iron treatment among women with anemia. This is the tip of the iceberg: Our new MapEnhance offering combines data from EMRs, facility chargemasters, and lab and precision molecular diagnostics, unlocking many possibilities for overcoming traditional data roadblocks at scale. The depth of this additional context will allow for new comprehensive analytics, higher-fidelity insights, and greater healthcare research on therapeutic areas that have been limited historically by low visibility. By leveraging data from a multitude of sources and focusing on relationships between disparate datasets, Komodo’s tools open up unprecedented possibilities for researchers, advocacy groups, and industry experts alike.
For more information about populations affected by low data visibility, check out our post: Data Challenges Can Limit Accurate Insights for Transgender Patients — But New Tools Can Help.
To see more articles like this, follow Komodo Health on Twitter, LinkedIn, or YouTube, and visit Insights on our website.






