For Rare Diseases With No Standardized Treatment Strategy, Insights Can Help Inform Next Steps in Patients’ Journeys

In collaboration with the Foundation for Sarcoidosis Research (FSR), Komodo Health analysts identified the most common treatment and disease trajectories for patients with sarcoidosis, a rare inflammatory disease that disproportionately affects Black communities.
Some rare diseases are especially difficult to research, understand, and treat. With the existing limitation of having fewer patients to contribute to research, complexities grow when patients present with differing symptoms, and when disease trajectories are highly variable. Such is the case with sarcoidosis, a rare inflammatory disease for which a cause is still unknown. Due to the gaps in understanding and the complex clinical picture this disease presents, treatments are limited. This isn’t good news for the fewer than 200,000 American patients estimated to be impacted by sarcoidosis. A multifaceted approach to research is critical to make advancements in this disease, and others like it — AI, and a platform like Komodo’s, may play an important role in uncovering needed insights for experts and patients.
Primarily affecting adults, and more common in women than men, sarcoidosis causes groups of inflammatory cells (called “granulomas”) to develop on one or more of the body’s organs, most commonly the lungs. Chemical exposures, autoimmune dysfunction, microorganisms, and genetics are some of the possible contributing factors being explored in the search for a cause. As a disease with variable symptomatology, it’s a hard one to diagnose. Between 5 and 10% of sarcoidosis patients develop an advanced form of the condition, where granulomas interfere with the structure and function of the organs. Currently, sarcoidosis is treated with lifestyle changes, steroids, numerous off-label treatments like immunologics and biologics, and surgery, depending on the severity and manifestation of the disease. Patients are monitored for flares and the development of new manifestations. There is currently no standardized treatment strategy in practice.
As part of our work with the Chan Zuckerberg Initiative’s Rare As One network, and in partnership with the Foundation for Sarcoidosis Research (FSR), we explored the treatment journey of advanced sarcoidosis patients to add to the limited literature on treatment pathways. As sarcoidosis disproportionately affects Black Americans, we also investigated any racial disparities in care. Here’s what we found:
Between 2018 and early 2022, the number of sarcoidosis patients being treated increased.
This highlights a need for better understanding of the disease, and standardization in care for patients. It may also indicate that existing sarcoidosis patients continued to seek treatment throughout the pandemic. Patients treated with glucocorticoids, immunosuppressants, and TNF-alpha antagonists (medications used to treat inflammation and related symptoms) trended upward over the course of these four years. This held true for both Black and White sarcoidosis patients.
The five states with the highest numbers of sarcoidosis patients were New York, Pennsylvania, California, Texas, and Florida, which may be largely due to the higher populations of those states. The healthcare organizations with the highest numbers of sarcoidosis healthcare encounters were all located in the Northeast, and included the Cleveland Clinic, the University of Michigan Hospitals and Health Centers, Temple University Hospital, Hospital of the University of Pennsylvania, and Henry Ford Hospital.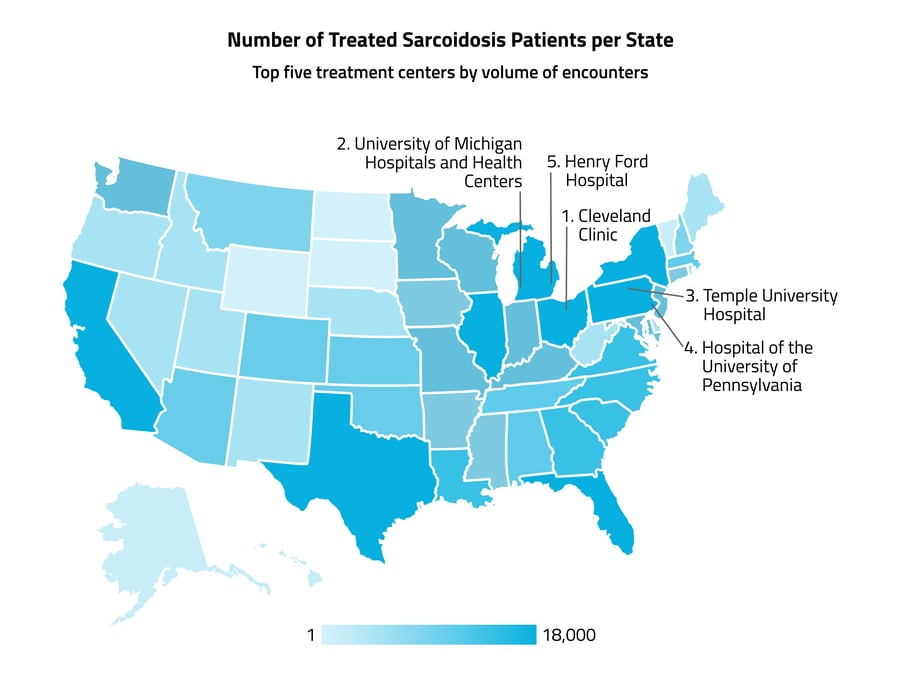
Black individuals had a significantly higher risk of developing sarcoidosis than White individuals.
We found that in 2021, 32% of patients newly diagnosed with sarcoidosis were Black and 62% were White (including those identifying as Hispanic White). Comparatively, only 13% of the American population is Black, while just under 76% are White or Hispanic White, according to the 2021 U.S. Census. These findings indicate a higher risk of developing sarcoidosis in the Black population compared with the White population.
In the treatment of advanced sarcoidosis, differences in the uses of branded vs. biosimilar drugs were present across commercial vs. Medicare/Medicaid insurance coverage.
In 2021, patients were more likely to be prescribed a higher-priced version of a TNF-a antagonist, Remicade, than the less expensive biosimilar drugs, such as Renflexis. While 68% were treated with Remicade, only 32% were treated with biosimilar drugs. Of sarcoidosis patients treated with the higher-priced Remicade, 23% were Black and 32% were White, while for Renflexis, 29% were Black and 26% were White. When looking at these differences by insurance type, patients with commercial coverage were more likely to get the brand-name drug, while patients on Medicare/Medicaid, of whom a larger percentage are Black than with commercial coverage, were more likely to get the biosimilar drug.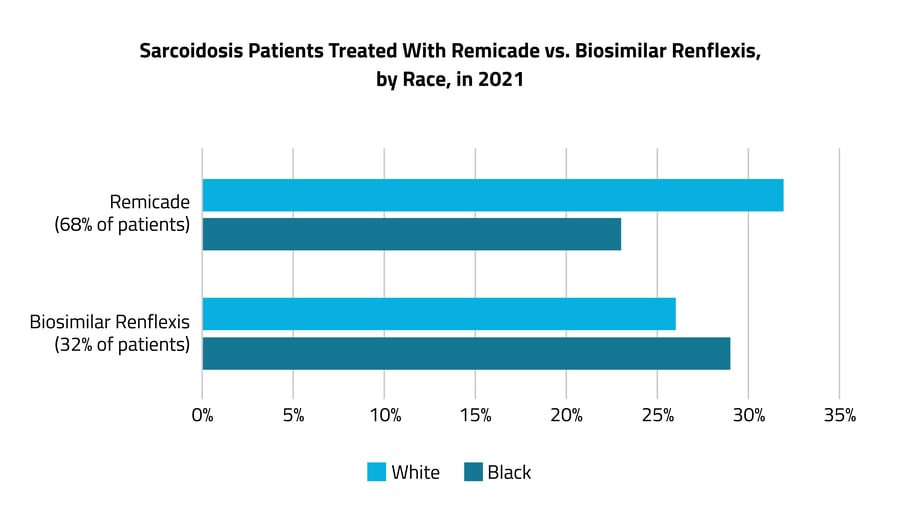 Off-label prescribing is common in the treatment of advanced sarcoidosis.
Off-label prescribing is common in the treatment of advanced sarcoidosis.
When lifestyle treatments aren’t effective, or when patients are first diagnosed with sarcoidosis that is already advanced, other treatments are necessary to manage symptoms and to prevent organ damage. In patients with advanced sarcoidosis, we found that the most common first step in treatment was with the steroid glucocorticoids prednisone, fluticasone, and furoate (which are typically on-label for sarcoidosis). If the patient’s disease failed to improve on steroids, the immunosuppressants mycophenolate, mofetil, and azathioprine were most commonly added as a second-line, off-label therapy. For patients with advanced sarcoidosis who had already taken a TNF-a antagonist (Infliximab or Adalimumab, also off-label), the most common next steps in treatment were chemotherapy or vedolizumab (a drug typically used to treat Crohn’s and Ulcerative Colitis).
For patients needing medical treatment, this typical use of off-label drugs is key. But this use also creates some of the current challenges in moving the needle in sarcoidosis, as lower visibility into treatments limits the understanding of patient responses to specific treatment protocols and their outcomes.
When the clinical guidelines of a disease are not standardized in practice or widely accepted, an understanding of common practices and their outcomes can help inform recommendations. Providers may benefit from this level of understanding as they must often rely on limited data and limited related professional training or support, with little visibility into peer approaches when treating a rare disease. Furthermore, patient journey visibility into second- and third-line therapies is needed — for the FDA and others — in order for treatments to advance, and in order to gain a representative understanding of the economic burden of various stages of this disease.
This analysis highlights the power that patient journey data and platforms like Komodo’s has to bring light to trends in the active treatment of rare diseases. Powered by over 330 million de-identified individual patient lives, Komodo’s tools are especially suited to provide rich and accurate insights in the rare disease space. In our mission to reduce the burden of disease, we will continue to collaborate with our partners to support providers with patient-level RWE and improve outcomes for all.
Authors:
Tabby Khan, MD, MPH, Medical Director, Komodo Health
Grace Travers, Customer Success Associate, Komodo Health
Tricha Shivas, MBe Chief Strategy Officer (CSO), Foundation for Sarcoidosis Research
Mary McGowan, CEO, Foundation for Sarcoidosis Research
Rebecca Epstein, MPH, Senior Research Manager, Foundation for Sarcoidosis Research
About FSR:
The Foundation for Sarcoidosis Research (FSR) is the leading international organization dedicated to finding a cure for sarcoidosis and improving care for sarcoidosis patients through research, education, and support. Since its establishment in 2000, FSR has fostered over $6 million in sarcoidosis-specific research efforts. To learn more, visit www.stopsarcoidosis.org
Read more about our work with 50+ rare disease advocacy groups through our partnership with the Chan Zuckerberg Initiative.







